New Advancements in the Study of the Liquid-Solid Interface of Ultrathin Ionic Liquid Films on Gold Surfaces
Introduction
Ionic liquids, which are salts with melting points below 100°C, composed of cations and anions, have gained widespread attention in various fields due to their unique physicochemical properties. In recent years, scientists have focused their attention on the interfaces between ionic liquids and their environments, such as liquid/liquid, liquid/gas, and liquid/solid interfaces, to explore their critical roles in multiphase reactions.
Latest Research Findings
A recent study from the Friedrich-Alexander-Universität Erlangen-Nürnberg in Germany has been published in the renowned journal Langmuir, attracting significant attention from the scientific community. The study, conducted by T. Cremer, F. Maier, and their team, is titled "Liquid/Solid Interface of Ultrathin Ionic Liquid Films: [C1C1Im][Tf2N] and [C8C1Im][Tf2N] on Au(111)." The researchers successfully deposited ultrathin films of two imidazolium-based ionic liquids ([C1C1Im][Tf2N] and [C8C1Im][Tf2N]) on Au(111) single-crystal surfaces using physical vapor deposition (PVD) techniques, and employed angle-resolved X-ray photoelectron spectroscopy (ARXPS) to monitor their adsorption behavior, orientation, and growth in real-time.
Key Findings
The in-depth investigation of these two ionic liquids revealed several interesting phenomena. First, the researchers observed that the anions and cations in the first layer of ionic liquid molecules directly contact the Au surface, forming a unique checkerboard-like arrangement. This arrangement provides a new perspective for understanding the interactions between ionic liquids and metal surfaces. Secondly, for [C8C1Im][Tf2N], the researchers found that as the coverage increases, the alkyl chains undergo reorientation, gradually moving away from the gold surface. This finding suggests that the orientation of ionic liquid molecules may change with the thickness of the thin film. Furthermore, through the analysis of the attenuation of the Au 4f signal, the study confirmed that these two ionic liquids form thin films on the Au(111) surface in a two-dimensional layer-by-layer growth mode, which is maintained up to a thickness of at least 9 nm (>10 ML). Finally, the research also discussed the impact of X-ray radiation on the ultrathin ionic liquid films, particularly the observed decomposition of the [Tf2N]−anion on the gold surface, providing important experimental evidence for understanding the beam damage effects.
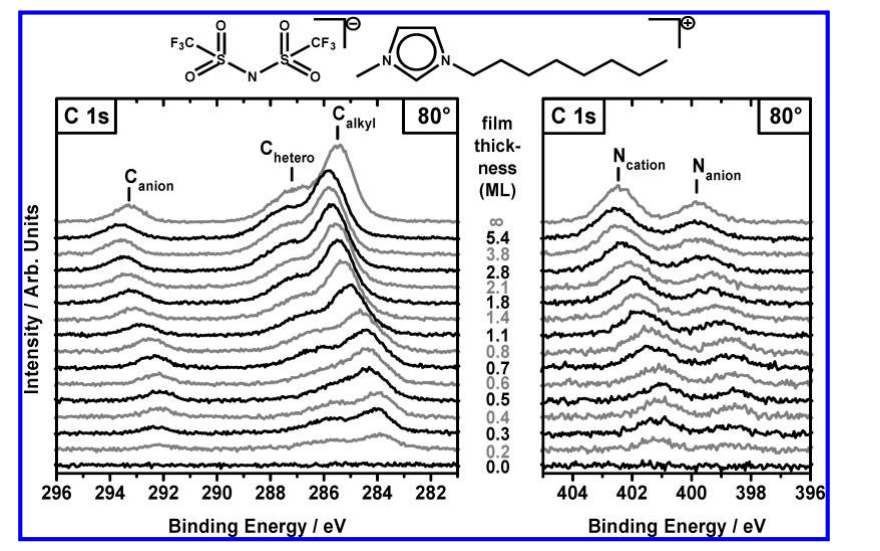
Waterfall plots of PVD films of [C8C1Im][Tf2N] on Au(111) with different thicknesses, starting from the clean substrate up to macroscopic
films. Depicted are the C 1s and N 1s core-level spectra for 80° emission.
Experimental Methods and Materials
In this study, the scientists utilized a crucible material called pyrolytic boron nitride (PBN) to evaporate the ionic liquids under ultra-high vacuum (UHV) conditions using physical vapor deposition (PVD) techniques. PBN, with its excellent high-temperature and chemical stability, has become an ideal choice for researchers handling organic materials under high-temperature conditions. Specifically, the researchers used a modified high-temperature Knudsen cell (Traectra WKC3) for ionic liquid evaporation, with the evaporation crucible part made of PBN.
During the experiments, approximately 1 ml of the target ionic liquids were placed in a PBN crucible that was adapted to the evaporator. To prevent bubbling of the ionic liquids during the evaporation process, the researchers slowly evacuated the setup to UHV conditions and maintained it overnight. Before the start of evaporation, the samples were held at temperatures about 30 K lower than the evaporation temperature and further degassed and purified for several hours under a base pressure of around 1 x 10^-9 mbar. Depending on the type of ionic liquid and the desired deposition rate, the evaporation temperature was precisely controlled between 410 K and 440 K. During the ionic liquid deposition and XPS measurements, the substrate temperature was maintained between 290 K and 320 K. To ensure a constant ionic liquid flux, the researchers also utilized a quartz crystal microbalance (QCM) for real-time monitoring of the evaporation rate.
Notably, QSAM Inc., as the leading manufacturer of PBN crucibles in the market, has provided high-quality services to researchers. Their professional manufacturing capabilities and customized services have enabled researchers to obtain the most suitable high-quality crucibles for their experimental needs, thus facilitating the advancement of scientific research.
PBN crucible
Significance and Outlook
This research provides new insights into the adsorption, arrangement, and growth behavior of ionic liquids on metal single-crystal surfaces, and confirms the layer-by-layer growth mode of ionic liquid thin films. These findings not only deepen our understanding of the interactions between ionic liquids and solid surfaces but also provide an important theoretical foundation for the development of new ionic liquid-based materials and the optimization of related processes. Additionally, the observed beam damage effects, particularly the decomposition of the [Tf2N]−anion on the gold surface, offer valuable experience and insights for future related studies.
In the future, as the research on the interfacial behavior of ionic liquids continues to deepen, we can expect to see more innovative applications of ionic liquids in areas such as catalysis, energy storage, and lubrication. This study not only showcases the unique behavior of ionic liquids on the Au(111) surface but also lays the groundwork for exploring their behavior on other metal or semiconductor surfaces. With the persistent efforts of scientists, ionic liquids are expected to shine in more fields, making important contributions to addressing the energy and environmental challenges facing humanity.
